2.
Pathogenicity
N.meningitidis can be present in the human nasopharynx without causing harm. However, when a person's immune system is lowered, the bacteria can become pathogenic (10).
(Figure1) [A] first, N.meningitidis colonises the nasopharynx. [B] then, the bacteria pass the epithelium. The passing of the epithelium is done by binding the bacteria with its pili to a protein on the epithelial cell. Pili are long hair-like extensions on the bacterial surface (11). This protein is called carcinoembryonic antigen-related cellular adhesion molecules (CEACAMs). This binding stimulates the endocytosis of N.meningitidis into the cell, enabling it to pass through the epithelium (12).
After colonisation, N.meningitidis uses several virulence factors (Figure 3) to invade the host's immune system.
Opa and Opc are part of the OM opacity proteins. Opc proteins are encoded by a single gene and solely expressed by N.meningitidis, whereas Opa proteins are proteins of N.meningitidis and N.gonorrhoeae and encoded by numerous genes (13). Opa proteins mediate with CEACAMs (13,14).
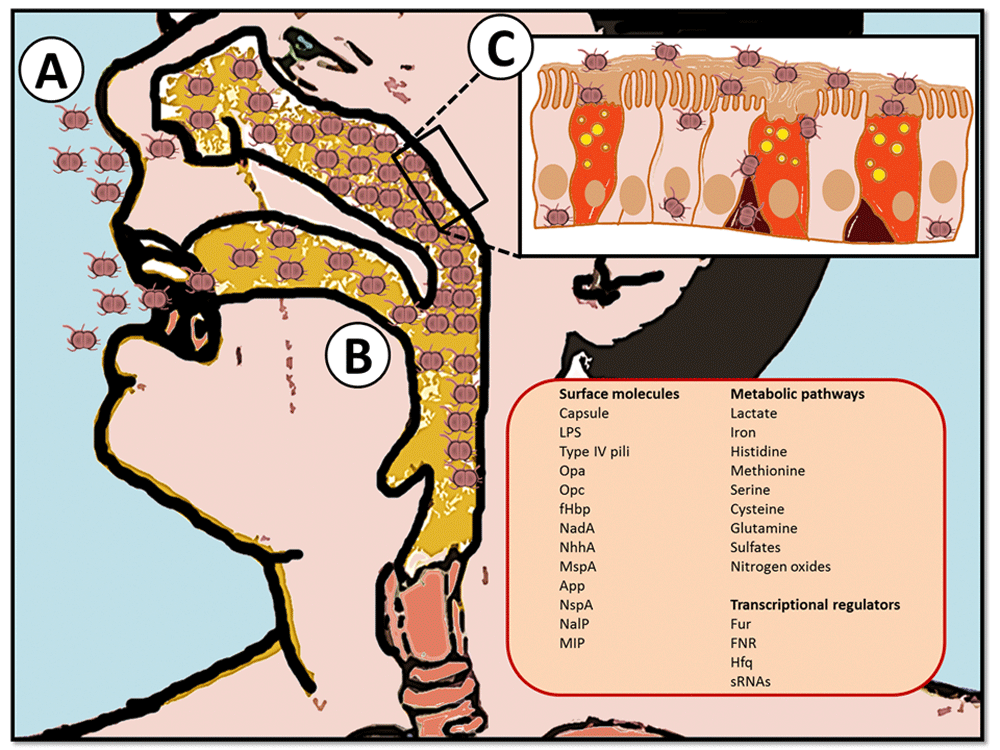
Figure 1: Graphical representation of N.meningitidis: Steps of colonisation (25).
When the bacteria invade the bloodstream, it is cable of evading the immune system and reaching meninge (Summarised in figure 2) (15).
The complement system is part of the immune system (15). It helps with inflammation and detects pathogens on their cell membrane. The body has several regulator proteins like factor H or C1q inhibitors to control this system. These regulator proteins can inhibit the system from making sure it doesn't overreact (16). N.meningitidis express factor H binding protein (fHBP). When N.meningitidis is covered in factor H, it cannot be destroyed by the complement system (17).
Multiple Steps in the Virulence of N.meningitidis expresses the lipooligosaccharides (LOS) structure (18). LOS is a lipid that can be bound by immune cells. Therefore, in the presence of N.meningitidis, the immune cells will alarm the immune system resulting in a response called sepsis. However, immune cells can only bind specific confirmations, meaning N.meningitidis can escape the immune system by changing the confirmation of LOS. The confirmation was changed by adding phosphate and phosphoethanolamine (PEA) (13).
Another virulence factor includes a polysaccharide capsule around the bacteria (19). This is an extra layer around the bacteria to prevent phagocytosis. Phagocytosis is when the bacteria is being absorbed by immune cells (20). The capsule prevents insertion of the membrane attack complex (MAC). MAC is an effector protein of the complement system to destroy pathogens. The capsule around N.meningitidis protects the bacteria from the insertion of the MAC, and therefore it can evade the complement system (17).
Once the bacteria are in the bloodstream, they can cross the blood-brain barrier. The vessels in the brain are lined with specialised endothelial cells that form a relatively tight barrier between the blood and brain tissue. These cells are more strongly connected through tight junctions and adherence junctions (21).
Furthermore, the B2 adrenergic receptor and CD147 serves as a particular docking site for the passing bacteria. This attachment allows them to rest on the surface of the endothelium (22). Thus, passing the blood-brain barrier, N.meningitidis starts to multiply uncontrollably. In addition, the interaction of bacterial components with meningeal cells initiates the production of cytokines, thus inflammation (13).

Figure 2: Stages in the pathogenesis of N.meningitidis (23).

Figure 3: Virulence factors of N.meningitidis (24).
Without these factors, N.meningitidis would not be able to colonise, invade, and ultimately cause the disease in the host.
Figure 4: Video showing the pathogenesis of N.meningitidis (26).
References:
10. Meyer SA, Kristiansen PA. Household transmission of Neisseria meningitidis in the meningitis belt. The Lancet Global Health. 2016 Dec;4(12):e885–6.
11. Merz AJ, Rifenbery DB, Arvidson CG, So M. Traversal of a Polarized Epithelium by Pathogenic Neisseriae: Facilitation by Type IV Pili and Maintenance of Epithelial Barrier Function. Molecular Medicine. 1996 Nov;2(6):745–54.
12. Kuespert K, Roth A, Hauck CR. Neisseria meningitidis Has Two Independent Modes of Recognizing Its Human Receptor CEACAM1. Ahmed N, editor. PLoS ONE. 2011 Jan 27;6(1):e14609.
13. Rouphael NG, Stephens DS. Neisseria meningitidis: Biology, Microbiology, and Epidemiology. Methods in Molecular Biology [Internet]. 2011 Sep 9 [cited 2021 Oct 29];799:1–20. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4349422/
14. Schubert-Unkmeir A. Molecular mechanisms involved in the interaction of Neisseria meningitidis with cells of the human blood–cerebrospinal fluid barrier. Pathogens and Disease. 2017 Mar 1;75(2).
15. Lewis LA, Ram S. Meningococcal disease and the complement system. Virulence [Internet]. 2014 Jan 1 [cited 2021 Oct 25];5(1):98–126. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3916388/
16. Tan LA, Yu B, Sim FCJ, Kishore U, Sim RB. Complement activation by phospholipids: the interplay of factor H and C1q. Protein & Cell. 2010 Nov;1(11):1033–49.
17. Kugelberg E, Gollan B, Tang CM. Mechanisms in Neisseria meningitidis for resistance against complement-mediated killing. Vaccine. 2008 Dec;26:I34–9.
18. Plant L, Sundqvist J, Zughaier S, Lövkvist L, Stephens DS, Jonsson A-B. Lipooligosaccharide Structure Contributes to Multiple Steps in the Virulence of Neisseria meningitidis. Infection and Immunity [Internet]. 2006 Feb 1 [cited 2021 Dec 26];74(2):1360–7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1360357/
19. Hsieh SA, Allen PM. Immunomodulatory Roles of Polysaccharide Capsules in the Intestine. Frontiers in Immunology [Internet]. 2020 Apr 15 [cited 2021 Oct 26];11:690. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7174666/
20. Johswich K. Innate immune recognition and inflammation in Neisseria meningitidis infection. Pathogens and Disease. 2017 Mar 1;75(2).
21. Coureuil M, Mikaty G, Miller F, Lécuyer H, Bernard C, Bourdoulous S, et al. Meningococcal Type IV Pili Recruit the Polarity Complex to Cross the Brain Endothelium. Science. 2009 Jun 11;325(5936):83–7.
22. Maïssa N, Covarelli V, Janel S, Durel B, Simpson N, Bernard SC, et al. Strength of Neisseria meningitidis binding to endothelial cells requires highly-ordered CD147/β2-adrenoceptor clusters assembled by alpha-actinin-4. Nature Communications [Internet]. 2017 Jun 1 [cited 2021 Oct 27];8:15764. Available from: https://pubmed.ncbi.nlm.nih.gov/28569760/
23. Virji M. Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nature Reviews Microbiology [Internet]. 2009 Apr [cited 2021 Nov 15];7(4):274–86. Available from: https://www.nature.com/articles/nrmicro2097
24. F Verheul A. TABLE 2 . Virulence factors of N. meningitidis [Internet]. ResearchGate. 1993 [cited 2021 Nov 15]. Available from: https://www.researchgate.net/figure/Virulence-factors-of-N-meningitidis_tbl2_14735718
25. Soriani M. Unraveling Neisseria meningitidis pathogenesis: from functional genomics to experimental models. F1000Research [Internet]. 2017 Jul 26 [cited 2021 Dec 23];6:1228. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5531161/
26. Hasudungan A. (Bacterial) Meningitis Pathophysiology [Internet]. YouTube. 2017 [cited 2021 Dec 23]. Available from: https://www.youtube.com/watch?v=xQC6L8M6XfU